Sustainability: Batteries & Solar | Water | Heating
Batteries | Consumption | Battery Recharging | Solar Charging | How Long Without Sun? | Alternate Charging Options | Solar Regulators | The Bottom Line
See also:
(Please note that this is a simplified description of a battery’s electrochemical process!)
 Batteries are devices that convert chemical energy into electrical energy.
Batteries are devices that convert chemical energy into electrical energy.
All batteries have a positive and negative terminal. The terminals on a 12V AGM auto battery are connected to six internal ‘cells’, each cell producing around 2.1 Volts. The cumulative output of all the cells connected together is around 12.6 Volts.
12V LiFePO4 (Lithium) batteries have four internal cells, each producing around 3.4V.
The Negative terminal of the battery is connected to internal plates within each cell known as the Anode, while the Positive terminal is connected to internal plates in each cell known as the Cathode.
The internal plates in each cell are separated by material known as the Electrolyte. In older lead-acid batteries this electrolyte was a liquid acid. In modern batteries the electrolyte is a paste, gel, or other non-liquid material. The electrolyte in AGM battery cells is absorbed in fibreglass mat.
When a battery is fully charged, the Anode has an excess of electrons, while the Cathode has a relative deficit of electrons. Nature being nature, the plates would like be ‘in balance’ and to have an equal number of electrons. Electrons are unable to move (without electrical stimulation) directly through the electrolyte to the opposite plate. Their only path to their preferred destination is via external wiring, which we cleverly contrive to take them through our appliances.
Accidentally connecting the negative terminal of the battery directly to the positive terminal with a conductive material, such as a steel spanner or a copper wire, is known as a short circuit. In such an instance, electrons from the Anode would move rapidly through the conductor to the Cathode in an attempt to reach their level of balance. Such a direct connection would create a heavy sparking current, possibly causing a fire, as the electrical current from the Anode ‘rushes’ to the Cathode.
This direct heavy flow of current can be disastrous for the battery and the conductor, and the device housing the battery. (This is where fuses come in!)
In reality we run the conductor path in such a way as to direct the electric current through, for example, a light bulb, in which case the light bulb will glow as the electrical current passes through on its way to the Cathode. The light bulb (or the associated circuitry) also provides resistance to a heavy flow of electrons from the Anode to the Cathode.
The same process applies for refrigerators, fans, and other Campervan and Motorhome appliances. Current moving from the battery’s Anode to the Cathode, via the appliance, is what makes these appliances function.
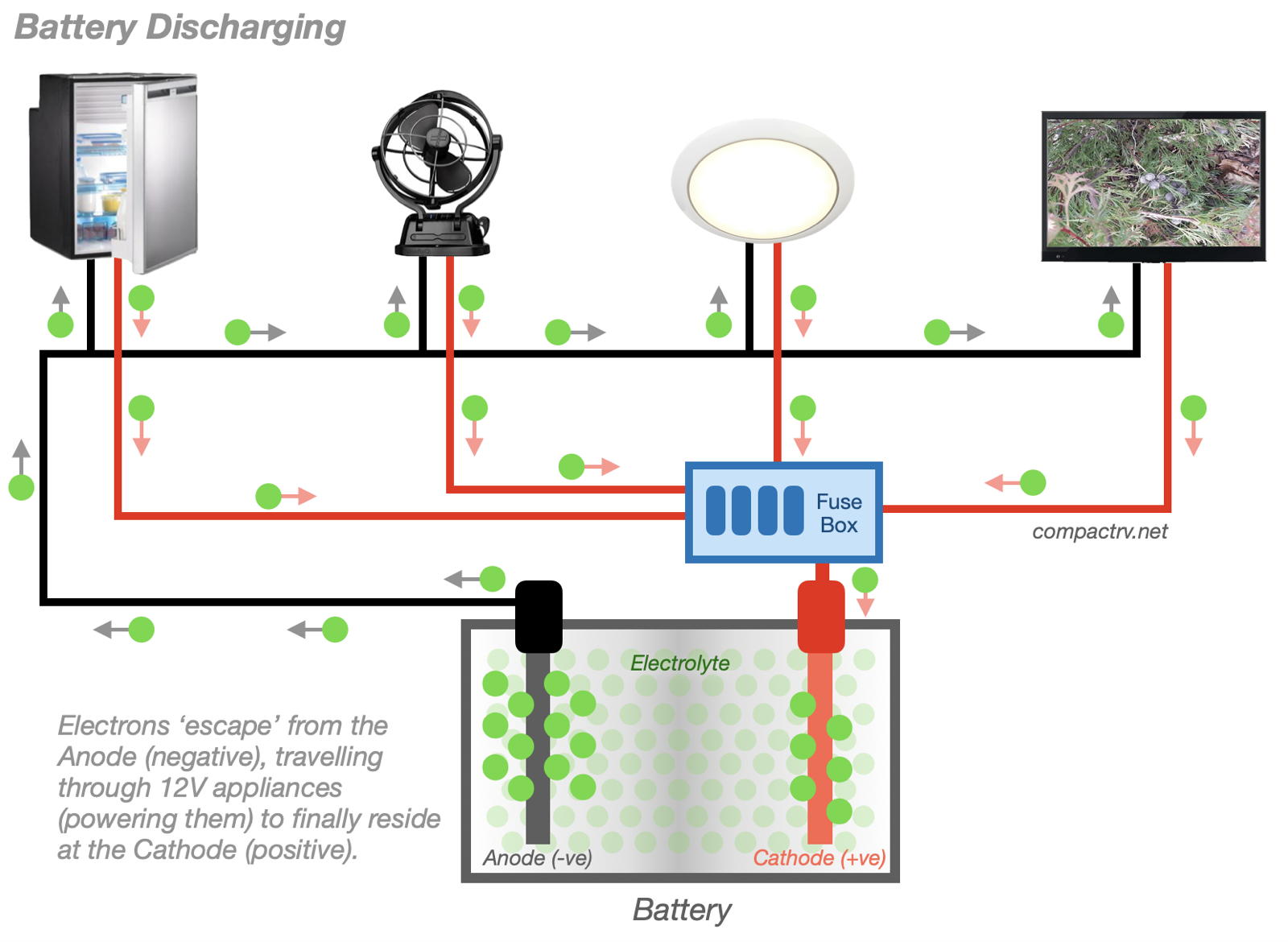
As the electrons move from the Anode to the Cathode, via our appliances, the battery is considered to be discharging. When the electrons on the Anode and Cathode are ‘in balance’, there is no longer an incentive for electrons to travel to the opposite terminal and the battery is considered to be discharged, or ‘flat’. (Electrons can move directly from the Anode to the Cathode through the Electrolyte, but at a very slow rate. This is how batteries go flat while in storage – a bigger issue for AGM batteries than for Lithium batteries.)
But all is not lost – if the battery is rechargeable.
The chemical make-up of the electrolyte in a rechargeable battery allows electrons to be ‘pushed’ from the Cathode back to the Anode. This requires an electrical current to be applied to the battery, via a battery charger which is able to adjust its current and voltage output to suit the chemistry of the battery, and change its output as the battery is recharged…..

The process of recharging requires a charging ‘profile’ with an adjustable input of voltage and current applied to the battery, depending on its state of charge. AGM and Lithium batteries require different charging ‘profiles’. Fortunately this is managed electronically by the battery charger.
The input of the battery charger is fed with electrical current from generating sources such as solar panels, the vehicle alternator, or a 230V mains power supply. As the state of charge of the battery is restored to its maximum potential, the output from the battery charger is tapered so as not to overcharge the battery.
Battery Chemistry
The materials used for the Anode, Cathode and Electrolyte can vary a little, but are generally from a similar family of chemicals….
AGM (Lead Acid) Batteries: The cathode is usually made of a lead dioxide alloy, while the anode is made of pure lead alloy. The electrolyte is sulfuric acid, embedded in fibreglass mesh. The use of lead alloy (a combination of lead with tin or calcium) improves the mechanical properties of the electrodes. On its own, lead is very soft and will deteriorate quickly.
Lithium (LiFePO4) Batteries: The cathode is made from Lithium Iron Phosphate, while the anode is made from graphitic carbon with a metallic backing. The electrolyte is usually a solution of lithium salts in a mixture of solvents (like dimethyl carbonate or diethyl carbonate).
|
More info about batteries and circuits….
|
See also:
Batteries | Consumption | Battery Recharging | Solar Charging | How Long Without Sun? | Alternate Charging Options | Solar Regulators | The Bottom Line
